Determination of Degree of Substitution of Sodium Carboxymethyl Cellulose
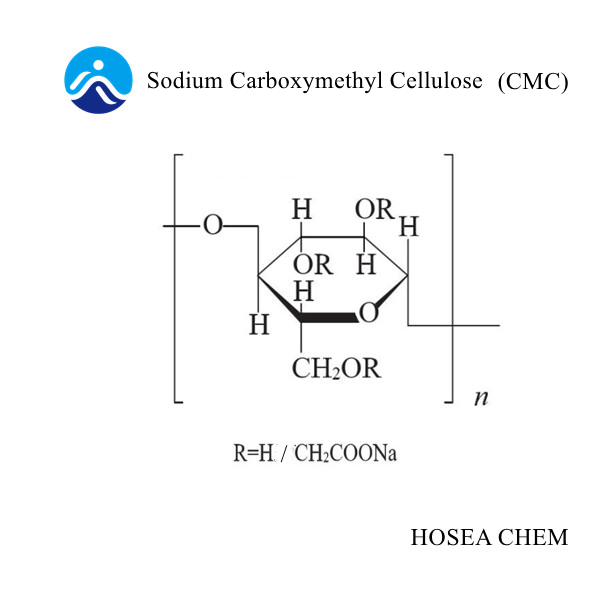
2021-12-13The molecular substitution DS of sodium carboxymethyl cellulose (CMC) is the average of the number of moles of sodium chloroacetate added to a glucose anhydride unit, so I think what you are asking is the degree of etherification of CMC.
Principle: Acidify water-soluble CMC to insoluble carboxymethyl cellulose. After purification, use accurately measured sodium hydroxide to convert a known amount of carboxymethyl cellulose into sodium salt, and then use hydrochloric acid standard solution Titrate the excess base.
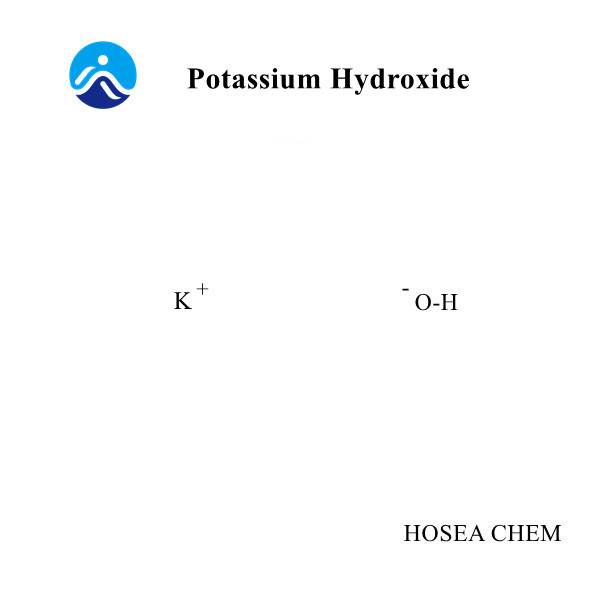
Reagents: 95 ethanol; 80 ethanol, anhydrous methanol; nitric acid; hydrochloric acid standard solution (0.4mol/L); sodium hydroxide standard solution (0.4mol/L); sulfuric acid (9 sulfuric acid: 2 water); diphenylamine reagent (0.5 g diphenylamine dissolved in 120ml sulfuric acid); phenolphthalein (1% ethanol solution)
Apparatus: Magnetic heating stirrer; beaker (250ml); conical flask (300ml); glass filter funnel (40ml, aperture 4.5-9um); 105 degree oven.
Operation:
1. Weigh 4g of sample in a beaker, add 75ml of 95% ethanol, stir it into a slurry with a laborious stirrer, add 5ml of nitric acid under stirring and continue to stir for 1-2min, heat and boil the slurry for 5min, stop heating , Continue to stir for 10-15min.
2. Pour the supernatant into the filter funnel, transfer the precipitate to the filter funnel with 100-150ml of 95% ethanol, and then wash the precipitate with 80% ethanol at 60°C until all the acid is removed.
3. Drop a few drops of filtrate from the filter funnel onto the white spotting plate, and add a few drops of diphenylamine reagent. If it is blue, it means that there is nitric acid and further washing is required.
4. Finally, wash the precipitate with a small amount of anhydrous methanol, continue to suction and filter until the methanol is completely removed, heat the oven to 105 degrees, turn off the electrogen, then put the filter funnel into the oven, open the door after 15 minutes to remove the methanol vapor, and close The oven door is connected to the power supply, dried at 105 degrees for 3 hours, and then cooled for 0.5 hours.
Etherification degree of sodium carboxymethyl cellulose in the sample:
A=(BC-DE)/F;
degree of etherification=0.162A/(1-0.058A)
A--The number of millimoles of sodium hydroxide consumed to neutralize 1g of carboxymethyl cellulose;
B--The volume of sodium hydroxide standard titration solution added, ml;
C--The concentration of sodium hydroxide standard solution, mol/L
D--The titration volume of the hydrochloric acid standard solution used to titrate the excess sodium hydroxide, ml;
E--Concentration of hydrochloric acid standard solution, mol/L
F--Used to determine the quality of acid carboxymethyl cellulose, g.
0.162-the millimolar mass of the dehydrated glucose unit of cellulose, g/mmol;
0.058--After a hydroxyl group in the dehydrated glucose unit is replaced by a carboxymethyl group, the net increase in the m molar mass of the dehydrated glucose unit, g/mmol.
Repeatability
The difference between the two determination results should not exceed 0.02 units of etherification degree