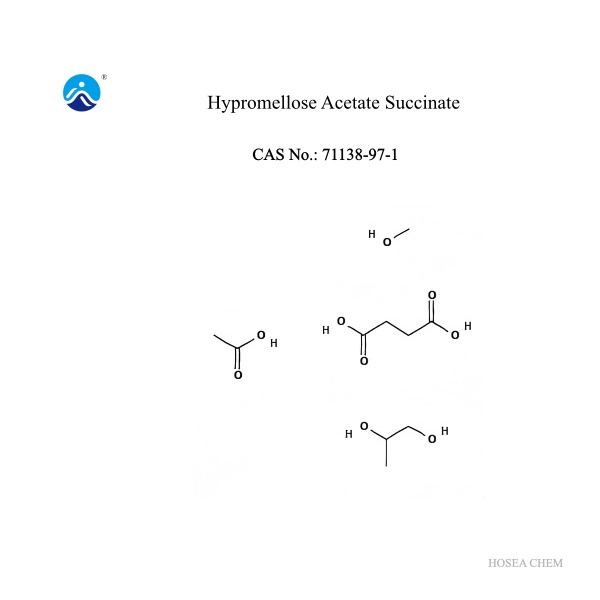
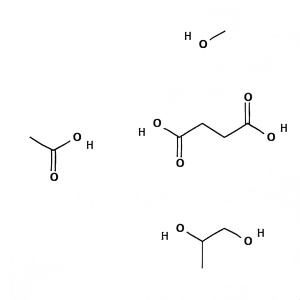
Hypromellose Acetate Succinate
Hosea Chem® has been supplying Hypromellose Acetate Succinate (HPMC-AS) with high quality and competitive price for many years, covering most of the European, American, etc. Send Inquiry
Product Description
Hypromellose Acetate Succinate
Specification of Product
Product Name : Hypromellose Acetate Succinate (HPMCAS)
Cas No.: 71138-97-1
Molecular formula: C10H22O9
Hypromellose Acetate Succinate Physical Data
L Grade M Grade H Grade
Viscosity 2.4-3.6
Free Acetylsuccinate,% ≤ 1.0
Loss on Drying,% ≤ 5.0
Residue on Ignition,% ≤ 0.20
Heavy Metals,% ≤ 0.001
Acetate Content,% 5.0-9.0 7.0-11.0 10.0-14.0
Succinate Content,% 14.0-18.0 10.0-14.0 4.0.-8.0
Methoxy Group,% 20.0-24.0 21.0-25.0 22.0-26.0
Hydroxypropoxy Group,% 5.0-9.0 5.0-9.0 6.0-10.0
Hypromellose Acetate Succinate Properties
This product is white slightly yellow powder or granules with a slight acetic acid odor and a bulk density of 1.27-1.30 g/cm3 (measured by a helium pycnometer). It can be well dissolved in solvents such as acetone, ethanol-dichloromethane, and acetone-ethanol-water.
Packaging, transportation, and storage
Package:25Kgs/Bag or Drum.
This product should be stored in a dry, light-proof indoor warehouse to prevent moisture.
During the storage, transportation and loading and unloading of this product, avoid rain; avoid direct sunlight; ensure that it is dry and the packaging bag is not damaged or contaminated by other items; do not mix or store with toxic or harmful items; do not store at high temperatures.
Hypromellose Acetate Succinate Applications
As an enteric coating material, this product is characterized by excellent film-forming properties, no need for plasticizers, and good solubility in the upper small intestine (duodenum). This enhances drug absorption in the small intestine. It can also be used as a polymer carrier, in microcapsules and microspheres for drug preparations, and in sustained- or controlled-release formulations.