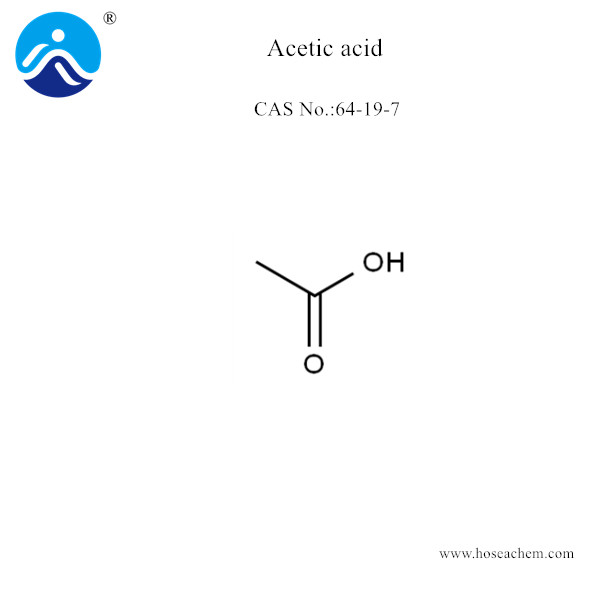
Acetic acid
Hosea Chem® has been supplying Acetic acid (CAS 64-19-7) with high quality and competitive price for many years, covering most of the European, American, etc. Send Inquiry
Product Description
Acetic acid
Chemical Name:Acetic acid;CAS 64-19-7
EINECS No.: 232-236-7
Chemical Formula: C2H4O2
Molecular Weight: 60.05
Melting point: 16.2°C
Boiling point: 117-118℃
Flash point: 104°F
Density at 25°C: 1.049g/mL
Molecular Structure:
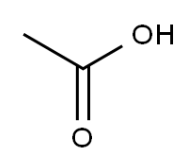
Description
Acetic acid, an organic compound, possesses several noteworthy characteristics. It manifests as a colorless and hygroscopic liquid, which intriguingly solidifies into ice-like crystals at lower temperatures. A distinctive attribute of this compound is its pungent smell. Furthermore, acetic acid exhibits intriguing behavior; it can be miscible with water, ethanol, ether, and carbon tetrachloride and remains insoluble in carbon disulfide. Caution is warranted when handling this substance due to its flammable and corrosive nature, evoking intense irritation.
Acetic acid Standard
Appearance: Colourless solution
Content %≥: 99.9
Density at 25°C: 1.049g/mL
Vapor pressure (20℃): 11.4 mmHg
Refractive index n20/D: 1.371
Application
1. It is mainly used for the preparation of acetic anhydride, vinyl acetate, acetic acid esters, metal acetate, chloroacetic acid, cellulose acetate, used in the production of ethyl acetate, edible spices, wine spices used as analytical reagents, solvents and lotion
2. Acetic acid occurs in vinegar. It is produced in the destructive distillation of wood. It finds extensive application in the chemical industry. It is used to manufacture cellulose acetate, acetate rayon, and various acetate and acetyl compounds; as a solvent for gums, oils, and resins; as a food preservative in printing and dyeing; and in organic synthesis. Glacial Acetic Acid is an acidulant that is a clear, colorless liquid with an acid taste when diluted with water. It is 99.5% or higher in purity and crystallizes at 17°c. It is used in salad dressings in a diluted form to provide the required acetic acid. It is used as a preservative, acidulant, and flavoring agent. It is also termed acetic acid, glacial. Acetic acid is used as table vinegar, a preservative, and an intermediate in the chemical industry, e.g., acetate fibers, acetates, acetonitrile, pharmaceuticals, fragrances, softening agents, dyes (indigo), etc. Product Data Sheet It is used in aqueous and non-aqueous acid-base titrations. Manufacture of various acetates, acetyl compounds, cellulose acetate, acetate rayon, plastics, and rubber in tanning; as laundry sour; printing calico and dyeing silk; as acidulant and preservative in foods; solvent for gums, resins, volatile oils, and many other substances. Widely used in commercial organic syntheses. Pharmaceutic aid (acidifier). Acetic acid is an important industrial chemical—the reaction of acetic acid with hydroxyl-containing compounds, especially alcohols, forms acetate esters. The most extensive use of acetic acid is in the production of vinyl acetate. Vinyl acetate can be produced through the reaction of acetylene and acetic acid. It is also made from ethylene and acetic acid. Vinyl acetate is polymerized into polyvinyl acetate (PVA), which produces fibers, films, adhesives, and latex paints. Cellulose acetate, used in textiles and photographic film, is produced by reacting cellulose with acetic acid and acetic anhydride in the presence of sulfuric acid. Other acetic acid esters, such as ethyl acetate and propyl acetate, are used in various applications. Acetic acid is used to produce plastic polyethylene terephthalate (PET). Acetic acid is used to produce pharmaceuticals.
Storge & Handling
Acetic Acid should be stored in a cool, dry, well-ventilated area away from direct sunlight, heat, and incompatible substances. It is advisable to store it in airtight containers made of materials such as stainless steel, glass, or high-density polyethylene. Acetic Acid should be handled with appropriate protective equipment, including gloves, goggles, and suitable clothing, as it can cause irritation and burns upon contact.
Packing
1T/Drum; 215KG/Drum