Methylene-Chloride-(Dichloromethane)
Hosea Chem® has been supplying Methylene Chloride (CAS 75-09-2) with high quality and competitive price for many years, covering most of the European, American, etc. Send Inquiry
Product Description
Methylene Chloride
Chemical Name:Methylene Chloride;Dichloromethane;CAS 75-09-2
EINECS No.: 200-838-9
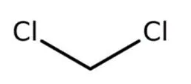
Chemical Formula: CH2CI2
Molecular Weight: 84.93
Melting point: -97°C
Boiling point: 39-40℃
Density: 1.325
Molecular Structure:
Description
1. Natural gas chlorination reaction of natural gas and chlorine, after absorption of hydrogen chloride by water and by-production of hydrochloric acid, the residual trace of hydrogen chloride is removed with alkali solution, and then dried, compressed, condensed and distilled to obtain the finished product.
2. Methyl chloride chlorination methyl chloride and chlorine in the 4000kw light reaction to generate methylene chloride, alkali washing, compression, condensation, drying and distillation to obtain the finished product. The main by-product was chloroform.
Maleic Anhydride Standard
Appearance: Colorless Transparent Liquid
Content %≥: 99.0
Density: 1.325
Saturated Vapor Pressure (kPa): 30.55 (10℃)
Ignition Temp. (℃): 615
Refractive index n20/D: 1.4242
Explosive limit %(v): 12-19
Application
Used as a solvent in the resin and plastic industries. Widely used in pharmaceutical, plastic and film industries. Dichloromethane has a wide range of applications. It is primarily used as a solvent in processes such as extraction, degreasing, and cleaning. It is also utilized as a paint stripper, a blowing agent in polyurethane foams, and an ingredient in pharmaceutical formulations.
Storge & Handling
Dichloromethane should be stored in tightly sealed containers in a cool, well-ventilated area, away from heat sources, ignition, and incompatible substances. Please keep it away from direct sunlight and open flames. Ensure proper labeling of containers, and store them separately from other chemicals to avoid potential reactions.
Packing
250KG/Drum